 |
| Zorotypus sp. photographed by Graham Montgomery in Texas |
 |
| A drone of Mastotermes darwiniensis |
 |
| A depiction of a Chulpex, painted by the illustrious Wayne Barlowe |
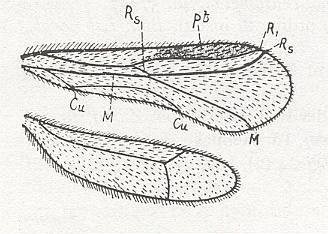 |
| Line drawing of the wings of an alate Zorotypus snyderi (Richards & Davies, 1977) |
Four hypotheses as to zorapteran phylogeny remain in the running today: first, that they comprise the adelphotaxon‡ of the clade Paraneoptera (Hennig, 1953; Beutel & Weide, 2005), which contains the orders Hemiptera (true bugs), lice–both free-living and parasitic (Psocodea), and Thysanoptera (thrips). The other three competing theories all place zorapterans in the Polyneoptera, which includes such familiar insects as cockroaches (Blattaria) and crickets (Orthoptera: Gryllidae). One posits that the Zoraptera are most akin to the sadly-misnamed earwigs (order Dermaptera) (Jarvis et al., 2005; Terry & Whiting, 2005). Another (Boudreaux, 1979; Smithers, 1991; Kukalová-Peck & Peck, 1993; Wheeler et al., 2001; Yoshizawa & Johnson, 2005; Wang et al., 2013) proposes that the superorder Dictyoptera (a well-supported group consisting of the orders Blattaria, Mantodea, and Isoptera) is the zorapteran sister-group.
 |
| Male (left) and female (right) webspinners (Oligotoma nigra) from New Mexico, photographed by Jim Mclarin |
 |
| Male (right) and female (left) Armillifer sp. tongue worms (Porocephalidae) |
Farewell, and beware the ides of March.
*Members of a winged morph.
†"Truly social."
‡Sister-group.
||No, I do not speak French.
__________________________________________________________
Abele, L. G.; Kim, W.; and Felgenhauer, B. E. (1989). Molecular evidence for the inclusion of the phylum Pentastomida in the Crustacea. Molecular Biology and Evolution, 6(6), 685-691. Retrieved 3/15/13 from http://mbe.oxfordjournals.org/content/6/6/685.full.pdf
Almeida, O. W.; Christofferson, M. L.; Amorim, D. S. and Eloy, E. C. C. (2008). Morphological support for the phylogenetic positioning of Pentastomida and related fossils. Biotemas, 21(3), 81-90.
van Beneden, P. J. (1849). Recherches sur l'organisation et le développment des linguatules (Pentastoma, Rud.) suivies de la description d'une espece nouvelle provenant d'un mandrill. Memoires de l'Academie de Bruxelles, serie 2, Zoologie, 23, 1-38.
Beutel, R. G. and Weide, D. (2005). Cephalic anatomy of Zorotypus hubbardi (Hexapoda: Zoraptera): new evidence for a relationship with Acercaria. Zoomorphology, 124, 121-136.
Boudreaux, H. B. (1979). Arthropod Phylogeny with Special Reference to Insects. John Wiley & Sons: New York.
Choe, J. C. (1994). Sexual selection and mating system in Zorotypus gurneyi Choe (Insecta, Zoraptera). II. Determinants and dynamics of dominance. Behavioral Ecology and Sociobiology, 34, 233-237.
Choe, J. C. (1997). The evolution of mating systems in the Zoraptera: Mating variations and sexual conflicts. In Choe, J. C. and Crespi, B. J. (eds.): The Evolution of Mating Systems in Insects and Arachnids (pp. 130-145). Cambridge University Press: Cambridge.
Dallai, R.; Mercati, D.; Gottardo, M.; Machida, R.; Mashimo, Y.; and Beutel, R. G. (2011). The male reproductive system of Zorotypus caudelli Karny (Zoraptera): sperm structure and spermiogenesis [electronic version]. Arthropod Structure & Development, 40(2011), 531-547. Retrieved 3/15/13 from http://www.researchgate.net/publication/51717799_The_male_reproductive_system_of_Zorotypus_caudelli_Karny_%28Zoraptera%29_Sperm_structure_and_spermiogenesis
Diesing, C. M. (1850). Systema Helminthum (vol. 1). Wilhelmum Braumüller: Vindobonae.
Engel, M. S. (2003). Phylogeny of the Zoraptera. Entomologische Abhandlungen, 61(2), 147-148.
Engel, M. S. (2005). Zoraptera. Tree of Life Web Project. Retrieved 3/15/13 from http://tolweb.org/Zoraptera/8252
Engel, M. S. and Grimaldi, D. A. (2006). The earliest webspinners (Insecta: Embiodea). American Museum Novitates. 15 pp., 2 figs., 3 tbs. Retrieved 3/14/13 from http://digitallibrary.amnh.org/dspace/bitstream/handle/2246/5791/N3514.pdf?sequence=1
Fröhlich, J. A. (1789). Beschreibungen einiger neuen Eingeweidewürmer. Der Naturforscher, 24, 101-162.
Grimaldi, D. and Engel, M. S. (2005). Evolution of the Insects. Cambridge: Cambridge University Press.
Hennig, W. (1953). Kritische Bemerkungen zum phylogenetischen System der Insekten. Beiträge zur Entomologie, 3, 1-85.
Heymons, R. (1935). Pentastomida. In Bronn, H. G. (ed.): Klassen und Ordnungen der Tierreichs (vol. 1) (pp. 1-267). Akadem Verlagsgesellschaft: Leipzig.
Hoell, H. V.; Doyen, J. T.; and Purcell, A. H. (1998). Introduction to Insect Biology and Biodiversity (2nd ed.). Oxford University Press: Oxford.
Imms, A. D. (1931). Social Behaviour in Insects. Read Books: London.
Jarvis, K. J.; Haas, F.; and Whiting, M. F. (2005). Phylogeny of earwigs (Insecta: Dermaptera) based on molecular and morphological evidence: reconsidering the classification of Dermaptera. Systematic Entomology, 30, 442-453.
Kristensen, N. P. (1991). Phylogeny of extant hexapods. In Naumann, I. D.; Cornell, P. B. C.; Lawrence, J. F.; Neilson, E. S.; Spradberry, J. P.; Taylor, R. W.; Whitten, M. J.; and Littlejohn, M. J. (eds.): The Insects of Australia: a Textbook for Students and Research Workers (2nd ed.) (pp. 125-140). CSIRO, Melbourne: Melbourne University Press.
Kukalová-Peck, J. and Peck, S. B. (1993). Zoraptera wing structures: evidence for new genera and relationship with the blattoid orders (Insecta: Blattoneoptera). Systematic Entomology, 18, 333-350.
Lavrov, D. V.; Brown, W. M.; and Boore, J. L. (2004). Phylogenetic position of the Pentastomida and (pan)crustacean relationships. Proceedings of the Royal Society of London B, 271(1538), 537-544. Retrieved 3/15/15 from http://www.eeob.iastate.edu/faculty/LavrovD/publications/PDF_files/pentastomida.pdf
Leuckart, R. (1860). Bau und Entwicklungsgeschichte der Pentastomen nach Untersuchungen besonders von Pentastomum taenioides und P. dendiculatum. C. F. Winterische Verlagshandung: Leipzig.
Martin, J. W. and Davis, G. E. (2001). An Updated Classification of the Recent Crustacea. Natural History Museum of Los Angeles County. Retrieved 3/15/13 from http://atiniui.nhm.org/pdfs/3839/3839.pdf
Minet, J. and Bourgoin, T. (1986). Phylogenie et classification des Hexapodes (Arthropoda). Cahiers Liaison, O.P.I.E. 20, 23-28.
Møller, O. S.; Oleson, J.; Avenant-Oldewage, A.; Thomsen, P. F.; and Glenner, H. (2008). First maxillae suction discs in Branchiura (Crustacea): development and evolution in light of the first molecular phylogeny of Branchiura, Pentastomida, and other "Maxillopoda". Arthropod Structure & Development, 37(4), 33-346.
Osche, G. (1963). Die Systematische Stellung und phylogenie der Pentastomida. Zeitschrift für Morphologie und Ökologie der Tiere, 52, 487-596.
Richards, O. W. and Davies, R. G. (1977). Imms' General Textbook of Entomology, vol. II (10th edition). Wiley: New York.
Rafael, J. A. and Engel, M. S. (2006). A new species of Zorotypus from Central Amazonia, Brazil (Zoraptera: Zorotypidae). American Museum Novitates, 3528, 1-11.
Ross, E. S. (2009). Embiidina. In Resh, V. H. and Cardé, R. T. (eds.): Encyclopedia of Insects (pp. 315-316). Academic Press: New York.
Self, J. T. (1969). Biological relationships of the Pentastomida; a bibliography of the Pentastomida. Experimental Parasitology, 24, 63-119.
Silvestri, F. (1913). Descrizione di un nuovo ordine di insetti. Bolletino del Laboratorio di Zoologia Generale e Agraria, 7, 193-209.
Smithers, C. N. (1991). Zoraptera. In Naumann et al. (eds.): Insects of Australia (pp. 410-411). Cornell University Press: Ithaca.
Summers, I. and Barlowe, W. D. (1979). Barlowe's Guide to Extraterrestrials. Workman Publishing Company: New York.
Terry, M. D. and Whiting, M. F. (2005). Mantophasmatodea and phylogeny of the lower neopterous insects. Cladistics, 21, 240-257.
Waloszek, D.; Repetski, J. E.; and Maas, A. (2006). A new Late Cambrian pentastomid and a review of the relationships of this parasitic group. Transactions of the Royal Society of Edinburgh: Earth Sciences, 96(2), 163-176.
Wang, Y.; Engel, M. S.; Rafael, J. A.; Dang, K.; Wu, H.; Wang, Y.; Xie, Q.; and Bu, W. (2013). A unique box in 28S rRNA is shared by the enigmatic insect order Zoraptera and Dictyoptera [electronic version]. PLoS ONE, 8(1), e53,679. Retrieved 3/13/13 from http://www.ncbi.nlm.nih.gov/pubmed/23301099
Wheeler, W. C.; Whiting, M.; Wheeler, Q. D.; and Carpenter, J. M. (2001). The phylogeny of the extant hexapod orders. Cladistics, 17, 113-169.
Wilson, E. O. (1959). Some ecological characteristics of ants in New Guinea rain forests. Ecology, 40, 437-447.
Wingstrand, K. J. (1972). Comparative spermatology of a pentastomid, Raillietiella hemidactyli, and a branchiuran crustacean, Argulus foliaceus, with a discussion of pentastomid relationships. Det Kongelige Danske Videnskabernes Selskab Biologiske Skrifter, 19(4), 1-72.
Yoshizawa, K. (2007). The Zoraptera problem: evidence for Zoraptera+Embiodea from the wing base. Systematic Entomology, 32, 197-204.
Yoshizawa, K. and Johnson, K. P. (2005). Aligned 18S for Zoraptera (Insecta): phylogenetic position and molecular evolution. Molecular Phylogenetics and Evolution, 37, 572-580.
Zrzavý, J. (2001). The interrelationships of metazoan parasites: a review of phylum- and higher-level hypotheses from recent morphological and molecular analyses. Folia Parasitologica, 48, 81-103. Retrieved 3/15/13 from http://faculty.uml.edu/rhochberg/hochberglab/Courses/Parasite/PDF%20Papers/Parasitology%20Papers/Interrelationships%20of%20Metazoan%20Parasites.pdf