 |
| Skeletal reconstruction of Godzilla as an oversized neoceratosaurian* (Carpenter, 1998) |
 |
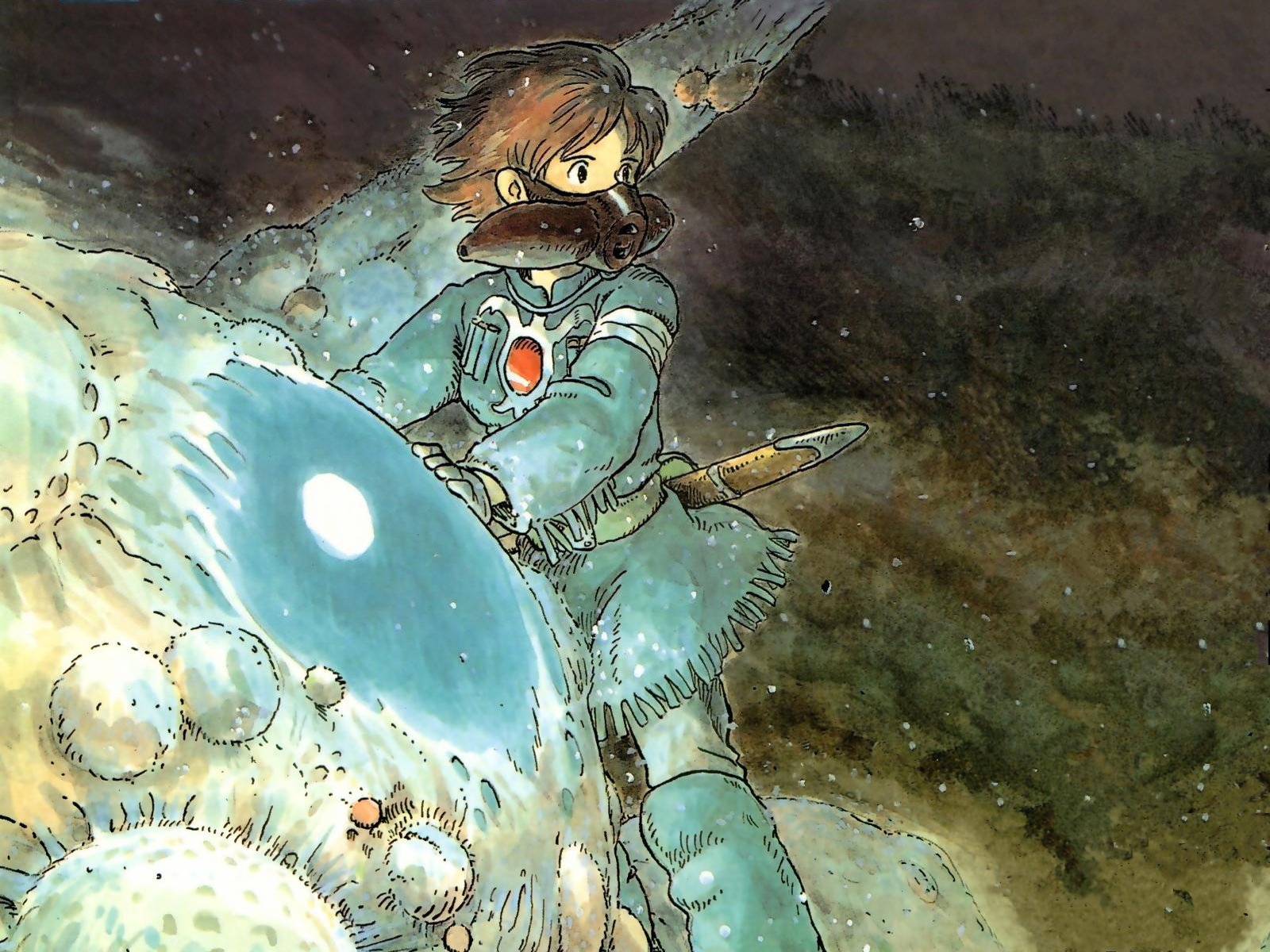
| Original theatrical poster for Nausicaä of the Valley of the Wind, by Yoshiyuki Takani |
 |
| Impression of the Sea of Corruption by Roberto Nieto |
At the manga's conclusion, it is revealed that the Sea of Corruption and all organisms therein were genetically engineered prior to the violent denouement of industrial civilization for the purpose of cleansing the biosphere (vol. 2, p. 441): autotrophs draw pollutants from the soil and metabolically render them insoluble solids; the Sea's gaseous effluvia is the byproduct of this process (Miyazaki, 2012). As a result, the Ohmu need not be pegged as the descendants of a factual organism, nor even conform to the bounds of plausibility under randomized evolutionary circumstances: indeed, it is probable that the Ohmu genome is a pastiche from multiple taxonomic sources. It only remains to determine which, exactly...
 |
| Nausicaä with molted Ohmu ocellus |
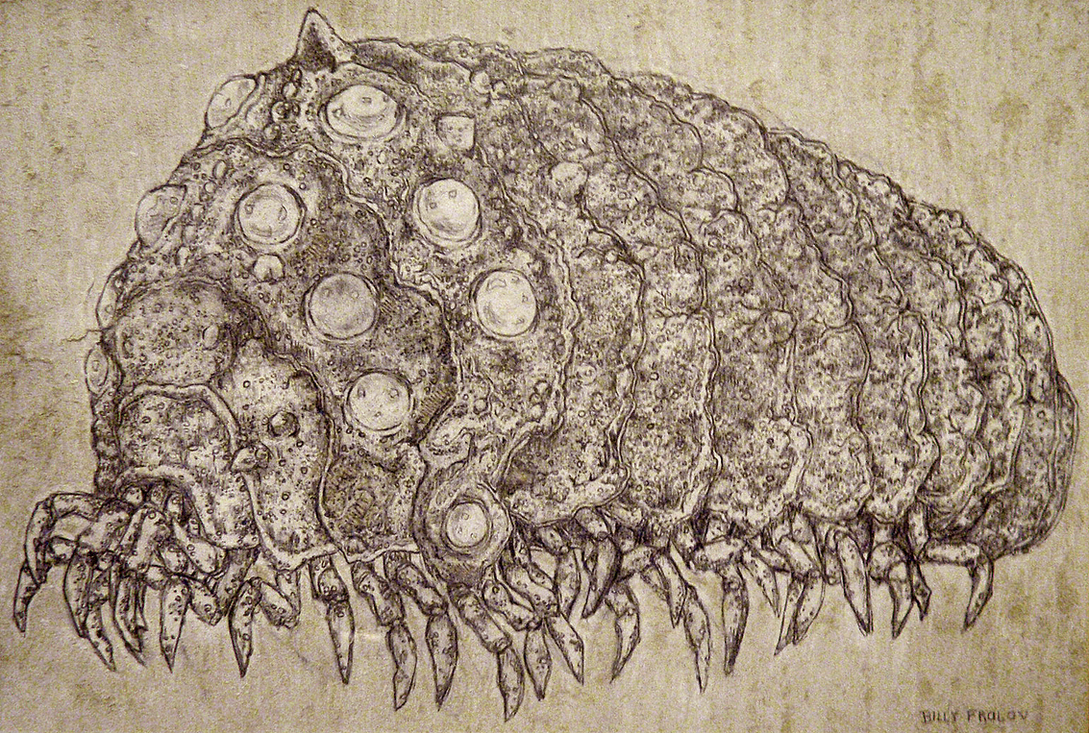 |
| Impression of an Ohmu by Billy Frolov |
 |
| Ventral view of Lepidurus apus (Notostraca: Triopsidae) by Ricardo Fernández |
 |
| Ohmu inspects a wounded Nausicaä with sensory tendrils |
 |
| Sphaerotheriid millipede, an oniscomorph |
 |
| Two Termitodesmus ceylonicus (Glomeridesmida: Termitodesmidae) captured by Rowland Shelley |
 |
| Depiction of assorted eurypterids by the dean of paleoartists, Charles R. Knight |
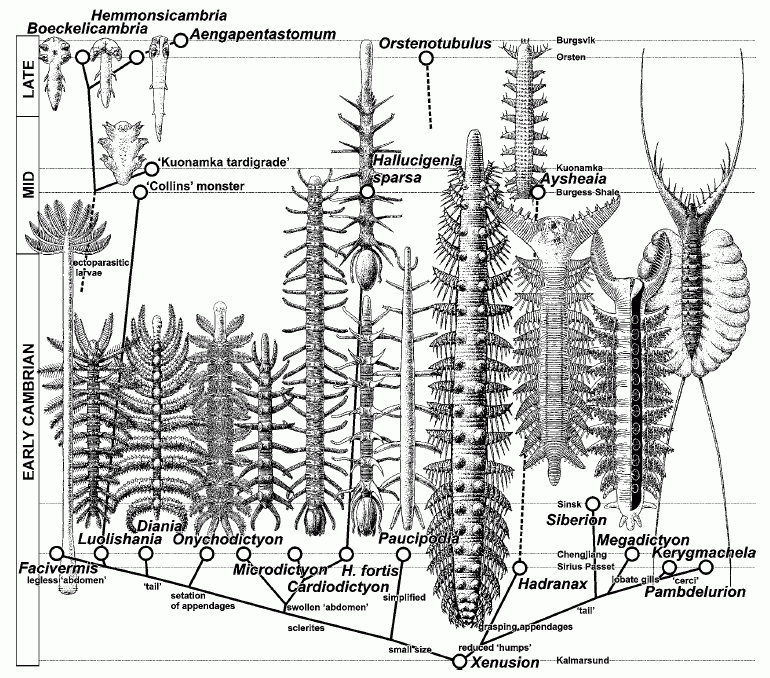 |
| Chronodendrogram of the paraphyletic lobopodian class Xenusia (Dzik, 2011) |
 |
| Pseudochalcura gibbosa (Eucharitidae), photographed by Kim Fleming |
 |
| Pissed Ohmu pursuing Yupa Miralda |
 |
| Nausicaä on Ohmu with epizoic growth |
In sum total, the only impossibility of Miyazaki's conception of the Ohmu is physiological: namely, their aforementioned gigantism. The largest known terrestrial arthropod in geological history was merely two meters in length, and only permitted to evolve such colossal dimensions by dint of the atmosphere it inhabited (14% more oxygenated than our contemporary air; Beerling, 2007): while the hydrosphere in Nausicaä's era is laden with pollutants, it is uncertain what residual effects humanity's ecological ruination has had upon atmospheric composition. Using Occam's Razor (or at least so I think), I propose that it is most likely that Ohmu respiration and circulation was genetically modified to facilitate their size.
...And now that we have exhausted the possibilities for intelligent discussion of the Ohmu, I consider this post finished.
*A clade consisting of the Abelisauroidea and Ceratosauridae (Pol & Rauhut, 2012).
†A ballpark estimate that remains in the realm of fanon, based upon the prologue's statement that industrial civilization collapsed roughly a millennium after its beginning, and that the story is set a millennium after that (Miyazaki, 2012); this is summed, of course, with a dating of the Industrial Revolution as commencing c. 1800 A.D.
‡Molted exoskeletons.
§Molting.
||The most inclusive group constituting the common ancestor of the phyla Arthropoda, Onychophora, and Tardigrada and all its descendants.
¶A morphological grade referring to primitive panarthropods too basal to lie within any of that clade's extant phyla.
_______________________________________________________________
Beerling, D. (2007). The Emerald Planet: How Plants Changed Earth's History. Oxford: Oxford University Press.
Blanke, A. and Wesener, T. (2014). Revival of forgotten characters and modern imaging techniques help to produce a robust phylogeny of the Diplopoda (Arthropoda, Myriapoda). Arthropod Structure & Development, 43(1), 63-75. Retrieved 8/16/15 from http://www.researchgate.net/publication/258252607_Revival_of_forgotten_characters_and_modern_imaging_techniques_help_to_produce_a_robust_phylogeny_of_the_Diplopoda_%28Arthropoda_Myriapoda%29
Carpenter, K. (1998). A dinosaur paleontologist's view of Godzilla. In Lees, J. D. and Cerasini, M. (eds.): The Official Godzilla Compendium (pp. 102-106). New York: Random House.
Darling, D. C. (1988). Comparative morphology of the labrum in Hymenoptera: the digitate labrum of Perilampidae and Eucharitidae (Chalcidoidea). Canadian Journal of Zoology, 66, 2811-2835. Retrieved 8/20/15 from http://www.researchgate.net/publication/238015432_Comparative_morphology_of_the_labrum_in_Hymenoptera_digitate_labrum_of_Perilampidae_and_Eucharitidae_%28Chalcidoidea%29
Drew, M. M.; Harzsch, S.; Stensmyr, M.; Erland, S.; and Hansson, B. S. (2010). A review of the biology and ecology of the Robber Crab, Birgus latro (Linnaeus, 1767) (Anomura: Coenobitidae). Zoologischer Anzeiger—A Journal of Comparative Zoology, 249(1), 45-67. Retrieved 8/24/15 from http://www.sciencedirect.com/science/article/pii/S0044523110000094
Dzik, J. (2011). The xenusian-to-anomalocaridid transition within the lobopodians. Bollettino della Società Paleontologica Italiana, 50 (1), 65-74. Retrieved 8/19/15 from http://palaeos.com/metazoa/ecdysozoa/panarthropoda/siberiidae.html#Siberiidae
Edgecombe, G. D. (2009). Palaeontological and Molecular Evidence Linking Arthropods, Onychophorans, and other Ecdysozoa. Evolutionary Education Outreach, 2, 178-190. Retrieved 8/19/15 from http://decapoda.nhm.org/pdfs/31062/31062.pdf
Enghoff, H. (1990). The ground plan of millipedes (external morphology). In Minelli, A. (ed.): Proceedings of the 7th International Congress of Myriapodology (pp. 1-22). Leiden: E. J. Brill.
Enghoff, H.; Dohle, W.; and Blower, J. G. (1993). Anamorphosis in millipedes (Diplopoda)—the present state of knowledge and phylogenetic considerations. Zoological Journal of the Linnaean Society, 109, 103-234.
Fusco, G. (2005). Trunk segment numbers and sequential segmentation in myriapods [electronic version]. Evolution & Development, 7(6), 608-617. Retrieved 8/12/15 from http://faculty.uml.edu/rhochberg/hochberglab/Courses/AdvancedInvertZool/Myriapoda/Fusco%202005.pdf
Garwood,
R. J. and Dunlop, J. (2014). Three-dimensional
reconstruction and the phylogeny of extinct chelicerate orders. PeerJ, 2, e641. Retrieved 8/17/15 from https://peerj.com/articles/641/
Gradstein, S. R.; Vitt, D. H.; and Anderson, R. S. (1984). The epizoic occurrence of Daltonia angustifolia (Musci) in Papua New Guinea. Cryptogamie: Bryologie et Lichénologie, 5, 47-50.
Hughes, N. C. (2003). Trilobite Tagmosis and Body Patterning from Morphological and Developmental Perspectives [electronic version]. Integrative & Comparative Biology, 43(1), 185-206. Retrieved 8/16/15 from http://icb.oxfordjournals.org/content/43/1/185.full
Jaenicke, E.; Pairet, B.; Hartmann, H.; and Decker, H. (2012). Crystallization and preliminary analysis of crystals of the 24-meric hemocyanin of the emperor scorpion (Pandinus imperator). PLoS One, 7(3), e32548. Retrieved 8/16/15 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3293826/
Koenemann, S.; Jenner, R. A.; Hoenemann, M.; and Stemme, T. (2010). Arthropod phylogeny revisited, with a focus on crustacean relationships. Arthropod Structure & Development, 39, 88-110. Retrieved 8/21/15 from http://decapoda.nhm.org/pdfs/31579/31579.pdf
Ma, X.; Edgecombe, G. D.; Legg, D. A.; and Hou, X. (2013). The morphology and phylogenetic position of the Cambrian lobopodian Diania cactiformis. Journal of Systematic Palaeontology, DOI: 10.1080/14772019.2013.770418. Retrieved 8/19/15 from http://www.researchgate.net/publication/51563234_Lobopodian_phylogeny_reanalysed
Machado, G. and Vidal, D. M. (2001). On the Occurrence of Epizoic Cyanobacteria and Liverworts on a Neotropical Harvestman (Arachnida: Opiliones). Biotropica, 33(3), 535-538. Retrieved 8/28/15 from http://www.jstor.org/stable/3593199?seq=1#page_scan_tab_contents
Martínez-Torres, S. D.; Daza, A. E. F.; and Linares-Costello, E. L. (2011). Meeting between kingdoms: discovery of a close association between Diplopoda and Bryophyta in a transitional Andean-Pacific forest in Colombia. International Journal of Myriapodology, 6, 29-36. Retrieved 8/25/15 from http://ijm.pensoft.net/articles.php?id=1917
Minelli, A. and Fusco, G. (2004). Evo-devo perspectives on segmentation: model organisms, and beyond. TREE, 19, 423-429.
Miyazaki, H. (2009). Starting Point: 1976-1996 (vol. 1). San Francisco: Viz Media.
Miyazaki, H. (2012). Nausicaä of the Valley of the Wind. San Francisco: Viz Media.
Miyazawa, H.; Ueda, C.; Yahata, K.; and Zhi-Hui, S. (2014). Molecular phylogeny of Myriapoda provides insights into evolutionary patterns of the mode in post-embryonic development. Scientific Reports, 4. Retrieved 8/11/15 from http://www.nature.com/srep/2014/140218/srep04127/full/srep04127.html
Naish, D. (2010). The science of Godzilla, 2010. Retrieved 8/22/15 from http://scienceblogs.com/tetrapodzoology/2010/11/01/science-of-godzilla-2010/
Pol, D. and Rauhut, O. W. M. (2012). A Middle Jurassic abelisaurid from Patagonia and the early diversification of theropod dinosaurs. Proceedings of the Royal Society B: Biological Sciences, 279(1804), 3,170-3,175. Retrieved 8/8/15 from http://rspb.royalsocietypublishing.org/content/279/1741/3170
Posnien, N.; Bashasab, F.; and Bucher, G. (2009). The insect upper lip (labrum) is a nonsegmental appendage-like structure. Evolution & Development, 11(5), 480-488. Retrieved 8/25/15 from http://onlinelibrary.wiley.com/doi/10.1111/j.1525-142X.2009.00356.x/abstract;jsessionid=E8306F9EF5DDEFC330ED9C29611E7885.f04t01
Racheboeuf, P. R.; Hannibal, J. T.; and Vannier, J. (2004). A new species of the diplopod Amynilispes (Oniscomorpha) from the Stephanian lagerstätte of Montceau-les-Mines, France. Journal of Paleontology, 78(1), 221-229. Retrieved 8/15/15 from http://jpaleontol.geoscienceworld.org/content/78/1/221.extract
Racheboeuf, P. R.; Vannier, J.; Schram, F. R.; Chabard, D.; and Sotty, D. (2008). The euthycarcinoid arthropods from Montceau-les-Mines, France: functional morphology and affinities. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 99, 11-25. Retrieved 8/23/15 from http://decapoda.nhm.org/pdfs/30398/30398.pdf
Schultz,
J. W. (2007). A phylogenetic analysis
of the arachnid orders based on morphological characters. Zoological Journal of the Linnean
Society, 150(2), 221-265. Retrieved
8/17/15 from http://onlinelibrary.wiley.com/doi/10.1111/j.1096-3642.2007.00284.x/abstract;jsessionid=7B0B85238F91D5E035773F29CA516DF4.f01t02
Vintaned, J. A. G.; Liñán, E.; and Zhuravlev, A. Y. (2011). A New Early Cambrian Lobopod-Bearing Animal (Murero, Spain) and the Problem of the Ecdysozoan Early Diversification. In Pontarotti, P. (ed.): Evolutionary Biology—Concepts, Biodiversity, Macroevolution and Genome Evolution (pp. 193-219). Berlin: Springer-Verlag. Retrieved 8/25/15 from https://books.google.com.au/books?id=R3-3k5yoRhoC&printsec=frontcover&hl=en#v=onepage&q&f=false
Vintaned, J. A. G.; Liñán, E.; and Zhuravlev, A. Y. (2011). A New Early Cambrian Lobopod-Bearing Animal (Murero, Spain) and the Problem of the Ecdysozoan Early Diversification. In Pontarotti, P. (ed.): Evolutionary Biology—Concepts, Biodiversity, Macroevolution and Genome Evolution (pp. 193-219). Berlin: Springer-Verlag. Retrieved 8/25/15 from https://books.google.com.au/books?id=R3-3k5yoRhoC&printsec=frontcover&hl=en#v=onepage&q&f=false
No comments:
Post a Comment