 |
| Tenomerga cinerea (Cupedidae; photographed by ophis) |
 |
| Live Tetraphalerus bruchi (Ommatidae) photographed by Adriana Marvaldi |
 |
| Depiction of tshekardocoleid Sylvacoleus sharovi |
 |
| We praise thee, Alex Wild, for thy likeness of M. debilis herewith presented |
 |
| Well, it still makes more sense than some college class flowcharts I've seen... |
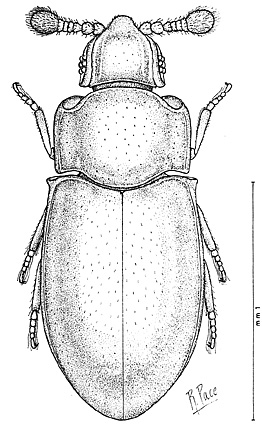 |
| Line drawing of Crowsoniella relicta (Pace, 1975) |
 |
| Holotype of Sikhotealinia zhiltzovae |
Jurodids present a confusing mosaic of traits: their enlarged coxae suggested that they should be placed within the Adephaga (Ponomarenko, 1985); whilst the discovery of the extant S. zhiltzovae (and additional fossil remains of Jurodes) revealed features that indicated an archostematan identity (Kirejtshuk, 1999) along with wing venation resembling that of the polyphagan superfamilies Scirtoidea and Scarabaeoidea (Fedorenko, 2009). Comparisons were therefore even drawn to the Derodontidae (which were later demonstrated as superficial; Ge et al., 2007; Yan et al., 2014), leading to the outright placement of S. zhiltzovae in the Polyphaga (Lafer, 1996). Strangest of all, though, is their possession of an ocellar triad on the head (Yan et al., 2014). Ocelli are absent in all other beetles (Leschen & Beutel, 2004) with the exception of a teratological omaliine rove beetle (Staphylinidae) (Naomi, 1987): even the Tshekardocoleidae lacked these light-sensing ommatidia (Ponomarenko, 2002). As a result, Jurodidae have been placed as the sister-group to the remaining Archostemata (Hörnschemeyer, 2005; Beutel et al., 2008).
Considering this phylogeny, notable atavism, and the fact that the Jurodidae have hardly changed since the mid-Jurassic (Yan et al., 2013) we can rightfully call them beetles that time forgot.
____________________________________________________________
Arnett, R. H. (1968). The Beetles of the United States. Ann Arbor: American Entomological Institute.
Barlet, J. (1996). Quelques précisions au sujet de Micromalthus (Insecta Coleoptera). Bulletin de la Société Royale des Sciences de Liège, 65, 373-378.
Beutel, R. G. and Hörnschemeyer, T. (2002a). Description of the larva of Rhipsideigma raffrayi (Coleoptera, Archostemata), with phylogenetic and functional implications. European Journal of Entomology, 99, 53-66.
Beutel, R. G. and Hörnschemeyer, T. (2002b). Larval morphology and phylogenetic position of Micromalthus debilis LeConte (Coleoptera: Micromalthidae) [electronic version]. Systematic Entomology, 27, 169-190. Retrieved 8/31/14 from http://onlinelibrary.wiley.com/doi/10.1046/j.1365-3113.2002.00172.x/pdf
Beutel, R. G.; Ge, S.-Q.; and Hörnschemeyer, T. (2008). On the head morphology of Tetraphalerus, the phylogeny of Archostemata and the basal branching events in Coleoptera. Cladistics, 24, 270-298. Retrieved 7/15/14 from http://www.researchgate.net/publication/229077348_On_the_head_morphology_of_Tetraphalerus_the_phylogeny_of_Archostemata_and_the_basal_branching_events_in_Coleoptera
Crowson, R. A. (1962). Observations on the beetle family Cupedidae, with the descriptions of two new fossil forms and a key to the recent genera. Annals of the Magazine of Natural History, 5, 147-157.
Crowson, R. A. (1975). The systematic position and implications of Crowsoniella. Bolletino del Museu Civico di Storia Naturale di Verona, 2, 459-463.
Crowson, R. A. (1981). The Biology of Coleoptera. London: Academic Press.
Fedorenko, D. N. (2009). Evolution of the Beetle Hind Wing, with Special Reference to Folding (Insecta: Coleoptera). Sofia-Moscow: Pensoft Publishers.
Foottit, R. G. and Adler, P. H. (2009). Insect Biodiversity: Science and Society. Hoboken: Wiley-Blackwell.
Frank, F.; Farrell, B. D.; and Beutel, R. G. (2009). The thoracic morphology of Archostemata and the relationships of the extant suborders of Coleoptera (Hexapoda). Cladistics, 25(1), 1-37. Retrieved 8/6/14 from http://dash.harvard.edu/bitstream/handle/1/3114645/farrell_thoracicmorphology.pdf?sequence=1
Ge, S.-Q.; Beutel, R. G.; and Yang, X. K. (2007). Thoracic morphology of adults of Derodontidae and Nosodendridae and its phylogenetic implications (Coleoptera). Systematic Entomology, 32, 635-667.
Ge, S.-Q.; Hörnschemeyer, T.; Friedrich, F.; and Beutel, R. G. (2010). Is Crowsoniella relicta really a cucujiform beetle? Systematic Entomology, 36(1), 175-179. Retrieved 7/22/14 from http://www.researchgate.net/publication/229077093_Is_Crowsoniella_relicta_really_a_cucujiform_beetle
Gradstein, F. M.; Ogg, J. G.; Schmitz, M.; and Ogg, G. (2012). A Geologic Time Scale 2012. Amsterdam: Elsevier B.V.
Hörnschemeyer, T. (2005). Archostemata Kolbe, 1908. In Kristensen, N. P. and Beutel, R. G. (eds.): Coleoptera, Vol. 1. Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga Partim). Handbook of Zoology Vol. IV, Arthropoda: Insecta (pp. 157-182). Berlin-New York: De Gruyter.
Hörnschemeyer, T. (2009). The species level phylogeny of archostematan beetles—where do Micromalthus debilis and Crowsoniella relicta belong? Systematic Entomology, 34(3), 533-558. Retrieved 7/22/14 from http://www.readcube.com/articles/10.1111/j.1365-3113.2009.00476.x
Hörnschemeyer, T. Tree of Life: Archostemata. (27 March, 2011). Retrieved 8/7/14 from http://tolweb.org/Archostemata/8876
Hörnschemeyer, T.; Wedmann, S. and Poinar, G. (2010). How long can insect species exist? Evidence from extant and fossil Micromalthus beetles (Insecta: Coleoptera) [electronic version]. Zoological Journal of the Linnean Society, 158, 300-311. Retrieved 8/814 from http://www.researchgate.net/publication/223131272_How_long_can_insect_species_exist_Evidence_from_extant_and_fossil_Micromalthus_beetles_%28Insecta_Coleoptera%29
Hubbard, H. G. (1878). Description of the larva of Micromalthus debilis LeC. Proceedings of the American Philosophical Society, 17, 666-668.
Hutchinson, G. E. (1959). Homage to Santa Rosalia or Why are There So Many Kinds of Animals [electronic version]? The American Naturalist, 93(870), 145-159. Retrieved 7/16/14 from http://www.jstor.org/discover/10.2307/2458768?uid=3739680&uid=2&uid=4&uid=3739256&sid=21104341432977
Jeannel, R. and Paulian, R. (1949). Les coleóptères. Grassè, P-P. (ed.): Traité de Zoologie, 771-1,077. Paris: Masson.
Jing-Jing, T.; Ren, D.; and Chung-Kun, S. (2006). Palaeogeography, palaeoecology and taphonomy of Jurassic-Cretaceous Cupedomorpha faunas from China. Mesozoic Terrestrial Ecosystems, 130-133. Retrieved 7/17/14 from http://www.docstoc.com/docs/19891688/palaeogeography-palaeoecology-and-taphonomy-of-jurassic
Kolbe, H. (1908). Mein System der Coleopteren. Zeitschrift für Wissenschaftliche Insekten-Biologie, 4, 116-123, 153-162, 219-226, 246-251, 286-294, 389-400.
Kirejtshuk, A. G. (1999). Sikhotealinia zhiltzovae Lafer, 1996—recent representative of the Jurassic coleopterous fauna (Coleoptera, Archostemata, Jurodidae). Proceedings of the Zoological Institute RAS, 281, 21-26.
Kirejtshuk, A. G.; Nel, A.; and Collomb, F.-M. (2010). New Archostemata (Insecta: Coleoptera) from the French Paleocene and Early Eocene, with a note on the composition of the suborder. Annales de la Société Entomologique de France, (n.s.); 46, 216-227.
Kirejtshuk, A. G.; Poschmann, M.; Prokop, J.; Garrouste, R.; and Nel, A. (2013). Evolution of the elytral venation and structural adaptations in the oldest Paleozoic beetles (Insecta: Coleoptera: Tshekardocoleidae). Journal of Systematic Paleontology, 1-26. Retrieved 7/22/14 from http://www.researchgate.net/publication/258221814_Evolution_of_the_elytral_venation_and_structural_adaptations_in_the_oldest_Palaeozoic_beetles_%28Insecta_Coleoptera_Tshekardocoleidae%29
Lafer, G. S. (1996). Family Sikhotealiniidae. In Ler, P. A. (ed.): Key to the Insects of the Russian Far East, vol. III, pt. 3 (pp. 390-396). Vladivostok: Dal'nauka.
Lawrence, J. F. (1982). Coleoptera. Parker, S. (ed.): Synopsis and Classification of Living Organisms (pp. 482-553). New York: McGraw-Hill.
Lawrence, J. F. (1991). Ommatidae (=Ommadidae, including Tetraphaleridae), Cupedidae (Archostemata) (=Cupesidae), Micromalthidae (Archostemata), Buprestidae (Buprestoidea) (incl. Schizopodidae), Zopheridae (Tenebrionoidea) (including Merycidae). Cerambycidae (Chrysomeloidea) (including Disteniidae, Hypocephalidae, Oxypeltidae, Parandridae, Spondylidae, Vesperiidae). Stehr, F. W. (ed.): Immature Insects, vol. 2 (pp. 298-302, 386-388, 518-519, 556-561). Dubuque: Kendall/Hunt Publishing Co.
Lawrence, J. F. (1999). The Australian Ommatidae (Coleoptera: Archostemata): new species, larva and discussion of relationships. Invertebrate Taxonomy, 13, 369-390.
Lawrence, J. F. and Newton, A. F. (1982). Evolution and classification of beetles. Annual Review of Technology and Systematics, 13, 261-290.
Lawrence, J. F. and Newton, A. F. (1995). Families and subfamilies of Coleoptera. In Pakaluk, J. and Slipinski, S. A. (eds.): Biology, Phylogeny & Classification of Coleoptera; vol. 1 (pp. 779-1,006). Warszawa: Muzeum i Institut Zoologii PAN.
LeConte, J. L. (1878). The Coleoptera of Michigan. Proceedings of the American Philosophical Society, 17, 613.
Leschen, R. A. B. and Beutel, R. G. (2004). Ocellar atavism in Coleoptera: plesiomorphy or apomorphy? Journal of Zoological Systematics and Evolutionary Research, 42, 63-69.
Marshall, A. T. and Thornton, I. W. B. (1963). Micromalthus (Coleoptera: Micromalthidae) in Hong Kong. Pacific Insects, 5, 715-720.
Naomi, S. I. (1987). Comparative morphology of the Staphylinidae and allied groups (Coleoptera, Staphylinoidea). Japanese Journal of Entomology, 55, 450-458.
Pace, R. (1975). An exceptional endogeous beetle: Crowsoniella relicta n. gen. n. sp. of Archostemata Tetraphaleridae from central Italy. Bolletino del Museo Civico di Storia Naturale, Verona; 2, 445-458.
Ponomarenko, A. G. (1985). Coleoptera. In Rasnitsyn, A. P. (ed.): Jurassic Insects of Siberia and Mongolia (pp. 47-87). Moscow: Nauka.
Ponomarenko, A. G. (2002). Beetles. Scarabaeida. In Rasnitsyn, A. P. (ed.): Late Mesozoic Insects of Eastern Transbaikalia (pp. 164-176). Moscow: Nauka.
Ponomarenko, A. G. (2003). Ecological evolution of beetles (Insecta: Coleoptera) [electronic version]. Acta Zoologica Cracovensia, 46, 319-328. Retrieved 7/15/14 from http://www.zin.ru/animalia/coleoptera/pdf/ponomarenko_2003.pdf
Pollock, D. A. and Normark, B. B. (2002). The life cycle of Micromalthus debilis LeConte (1878) (Coleoptera: Archostemata: Micromalthidae): historical review and evolutionary perspective [electronic version]. Journal of Zoological Systematics and Evolutionary Research, 40, 105-112. Retrieved 8/31/14 from http://onlinelibrary.wiley.com/doi/10.1046/j.1439-0469.2002.00183.x/pdf
Rohdendorf, B. B.; Becker-Migdisova, E. E.; Martynova, O. M.; and Sharov, A. G. (1961). Paleozoic insects of Kuznetsky Basin. Trudy Palaeontologicheskogo Instituta AN SSSR, 85, 412-463.
Scott, A. C. (1938). Paedogenesis in the Coleoptera. Zeitschrift Morphologie Ökologie Tiere, 33, 633-653.
Smith, S. G. (1971). Parthenogenesis and polyploidy in beetles. American Zoology, 11, 341-349.
Tan, J.; Wang, Y.; Ren, D.; and Yang, X. (2012). New fossil species of ommatids (Coleoptera: Archostemata) from the middle Mesozoic of China illuminating the phylogeny of Ommatidae. BMC Evolutionary Biology, 12(113), 1-19. Retrieved 7/15/14 from http://www.biomedcentral.com/content/pdf/1471-2148-12-113.pdf
Tan, J.; Ren, D.; Shih, C.; and Yang, X. (2013). New schizophorid fossils from China and possible evolutionary scenarios for archostematan beetles. Journal of Systematic Paleontology, 11(1), 47-62. Retrieved 7/15/14 from http://www.tandfonline.com/doi/abs/10.1080/14772019.2011.637515#.U8RUv0CUW2k
White, M. J. D. (1973). Animal Cytology and Evolution (3rd. ed.). Cambridge: Cambridge University Press.
Yan, E. V.; Wang, B.; Ponomarenko, A. G.; and Zhang, H. (2014). The most mysterious beetles: Jurassic Jurodidae (Insecta: Coleoptera) from China. Gondwana Research, 25, 214-225. Retrieved 12/20/14 from http://www.researchgate.net/publication/259157836_The_most_mysterious_beetles_Jurassic_Jurodidae_%28Insecta_Coleoptera%29_from_China