 |
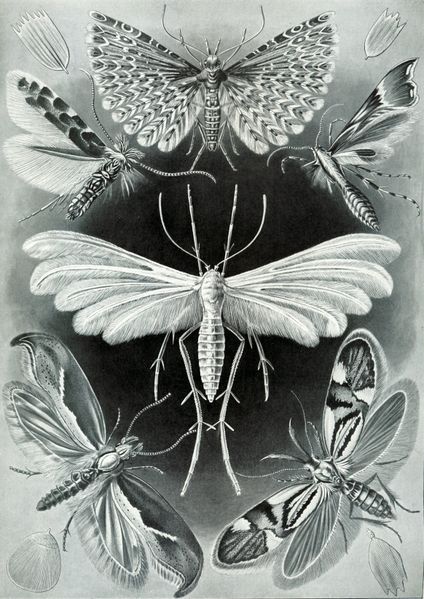
| Lithograph by Ernst Haeckel displaying moths of the families Plutellidae, Alucitidae, and Pterophoridae |
 |
| Tinagma gaedikei (Douglasiidae), known only in association with Miami mist (Phacelia purshii) (©microleps) |
 |
| Eupithecia orichloris (Geometridae) grappling with what appears to be a staphylinid beetle |
 |
| Euclemensia bassettella photographed by Mark Dreiling |
This taxonomic artifact is at odds with what is observed in another mega-diverse insect order with a scarcity of parasitoids: the Coleoptera (beetles). Only one parasitoidal lineage of these famously speciose elytron-bearers is ranked at or below generic level: namely, the genus Aleochara (Staphylinidae: Aleocharinae), which with the exception of one species, are larval ectoparasitoids of cyclorrhaphous fly pupae (Peschke & Fuldner, 1977; Peschke et al., 1996). Contrastingly, there are only two exclusively parasitoidal lepidopteran families: the related Epipyropidae and Cyclotornidae (both classified within the superfamily Zygaenoidea).
 |
| Final-instar caterpillar of Fulgoraecia exigua on acanaloniid; photographed by Gary McClellan |
Cyclotornids (consisting of five Australian species within the genus Cyclotorna; Common, 1990) exhibit strong ontogenetic parallels with the Epipyropidae. Female moths spread their eggs adjacent to ant trails (in Cyclotorna monocentra, those created by the dolichoderine Iridomyrmex purpureus); the miniscule first-instar larvae (of similar proportions to their epipyropid cousins) literally gallop along these thoroughfares (Common, 1990), which lead to aggregations of leafhoppers (Cicadellidae) farmed by I. purpureus. These cicadellids serve as initial hosts, the cyclotornid larvae oriented upon them similarly to epipyropids upon planthoppers (situated upon the abdomen below the wings): later instars (flattened and broad by comparison to the first) detach from their leafhopper hosts: exuding allomones attractive to the leafhopper-attending ants, the larvae are then borne back to the I. purpureus colony, where they reside as myrmecophiles; appeasing their hosts chemically whilst preying on the latter's brood before pupation in what is one of the more complicated ecological transitions any insect undergoes through metamorphosis (Dodd, 1912).
To conclude, not all the Lepidoptera are so uninteresting (or so deeply understood) as their repute would lead us to believe.
*The Macrolepidoptera is a probably monophyletic (Minet, 1991) clade including not only the butterflies (Papilionoidea) and the related skippers (Hesperioidea), but also such familiar moth families as the Geometridae (inchworms), Sphingidae (sphinx moths), the diverse Noctuidae (owlet moths and others), among many.
†Adult.
‡That is, parasitoids only when immature.
_____________________________________________________________
Common, I. F. B. (1990). Moths of Australia. Melbourne: Melbourne University Press.
Covell, C. V. (Jr.) (2005). A Field Guide to Moths of Eastern North America. Martinsville: Virginia Museum of Natural History.
Davis, D. R.; Quintero A., D.; Cambra T., R. A.; and Aiello, A. (2008). Biology of a new Panamanian bagworm moth (Lepidoptera: Psychidae), and eggs individually wrapped in setal cases [electronic version]. Annals of the Entomological Society of America, 101(4), 689-702. Retrieved 5/18/14 from http://esa.publisher.ingentaconnect.com/content/esa/aesa/2008/00000101/00000004/art00002
DeVries, P. J.; Chacon, I. A.; and Murray, D. (1992). Toward a better understanding of host use and biodiversity in riodinid butterflies (Lepidoptera). Journal of Research on the Lepidoptera, 31(1-2), 103-126.
Dodd, F. P. (1912). Some remarkable ant-friend Lepidoptera. Transactions of the Entomological Society of London, 1911, 577-590.
Fiedler, K. (2012). The host genera of ant-parasitic butterflies: a review. Psyche, 153975, 1-10. Retrieved 5/16/14 from http://www.hindawi.com/journals/psyche/2012/153975/
Gaston, K. J. (1991). The magnitude of global species richness. Conservation Biology, 5, 283-296.
Harrison, T. L. (2005). A new species of Douglasiidae (Lepidoptera) from the eastern Nearctic [electronic version]. Proceedings of the Entomological Society of Washington, 107(3), 596-603. Retrieved 5/16/14 from http://www.biodiversitylibrary.org/page/32143328#page/608/mode/1up
Hodges, A.; Hodges, G.; and Espelie, K. E. (2003). Parasitoids and parasites of Polistes metricus Say (Hymenoptera: Vespidae) in northeast Georgia. BioOne, 96(1), n.p.
Kirkpatrick, T. W. (1947). Notes on a species of Epipyropidae (Lepidoptera) parasitic on Metaphaena species (Hemiptera: Fulgoridae) at Amani, Tanganyika [electronic version]. Proceedings of the Royal Entomological Society of London, Series A: General Entomology; 22(4-6), 61-64. Retrieved 5/25/14 from http://onlinelibrary.wiley.com/doi/10.1111/j.1365-3032.1947.tb01108.x/abstract
Kristensen, N. P.; Scoble, M. J.; and Karsholt, O. (2007). Lepidoptera phylogeny and systematics: the state of inventorying moth and butterfly diversity [electronic version]. Zootaxa, 1668, 699-747. Retrieved 5/16/14 from http://www.lepidoptera.ee/images/lingid/Zootaxa1668p699.pdf
Mann, J. (1969). Cactus-feeding insects and mites. USNM Bulletin, 256, 30-158. Retrieved 5/17/14 from http://archive.org/stream/bulletinunitedst2561969unit#page/158/mode/2up
Marshall, A. T.; Lewis, C. T.; and Parry, G. (1974).
Montgomery, S. L. (1983). Carnivorous caterpillars: the behavior, biogeography and conservation of Eupithecia (Lepidoptera: Geometridae) in the Hawaiian Islands [electronic version]. GeoJournal, 7(6), 549-556. Retrieved 5/16/14 from http://link.springer.com/article/10.1007%2FBF00218529
Minet, J. (1991). Tentative reconstruction of the ditrysian phylogeny (Lepidoptera: Glossata). Entomologica Scandinavica, 22, 69-95.
Neunzig, H. H. (1996). The Moths of North America North of Mexico, fasc. 15.4.: Phycitinae (part). Bakersfield: the Wedge Entomological Research Foundation.
Perkins, R. C. L. (1905). Leaf-hoppers and their natural enemies (Pt. II: Epipyropidae) Lepidoptera. Bulletin of the Hawaiian Sugar Planter's Association Experimental Station Entomological Series, 84(1), 75-85.
Peschke, K. and Fuldner, D. (1977). Uebersicht und neue Untersuchungen zur Lebensweise der parasitoiden Aleocharinae (Coleoptera; Staphylinidae). Zoologische Jahrbuecher (Systematik), 104, 242-262.
Peschke, K.; Mittmann, B.; and Fuldner, D. (1996). Aleochara clavicornis, a stepping stone in the evolution of parasitoid behavior within the rove beetle genus Aleochara (Coleoptera, Staphylinidae). Proceedings of the XXth International Congress of Entomology, Firenze, August 25-31.
Pierce, N. E. (1995). Predatory and parasitic Lepidoptera: carnivores living on plants. Journal of the Lepidopterists' Society, 49, 412-453.
Rau, P. (1941). Observations on certain lepidopterous and hymenopterous parasites of Polistes wasps [electronic version]. Annals of the Entomological Society of America, 34(2), 355-366. Retrieved 5/21/14 from http://www.researchgate.net/publication/233678369_OBSERVATIONS_ON_CERTAIN_LEPIDOPTEROUS_AND_HYMENOPTEROUS_PARASITES_OF_POLISTES_WASPS
Watson, L. and Dallwitz, M. J. (2011). Blastobasidae. Retrieved 5/21/14 from http://delta-intkey.com/britin/lep/www/blastoba.htm
No comments:
Post a Comment